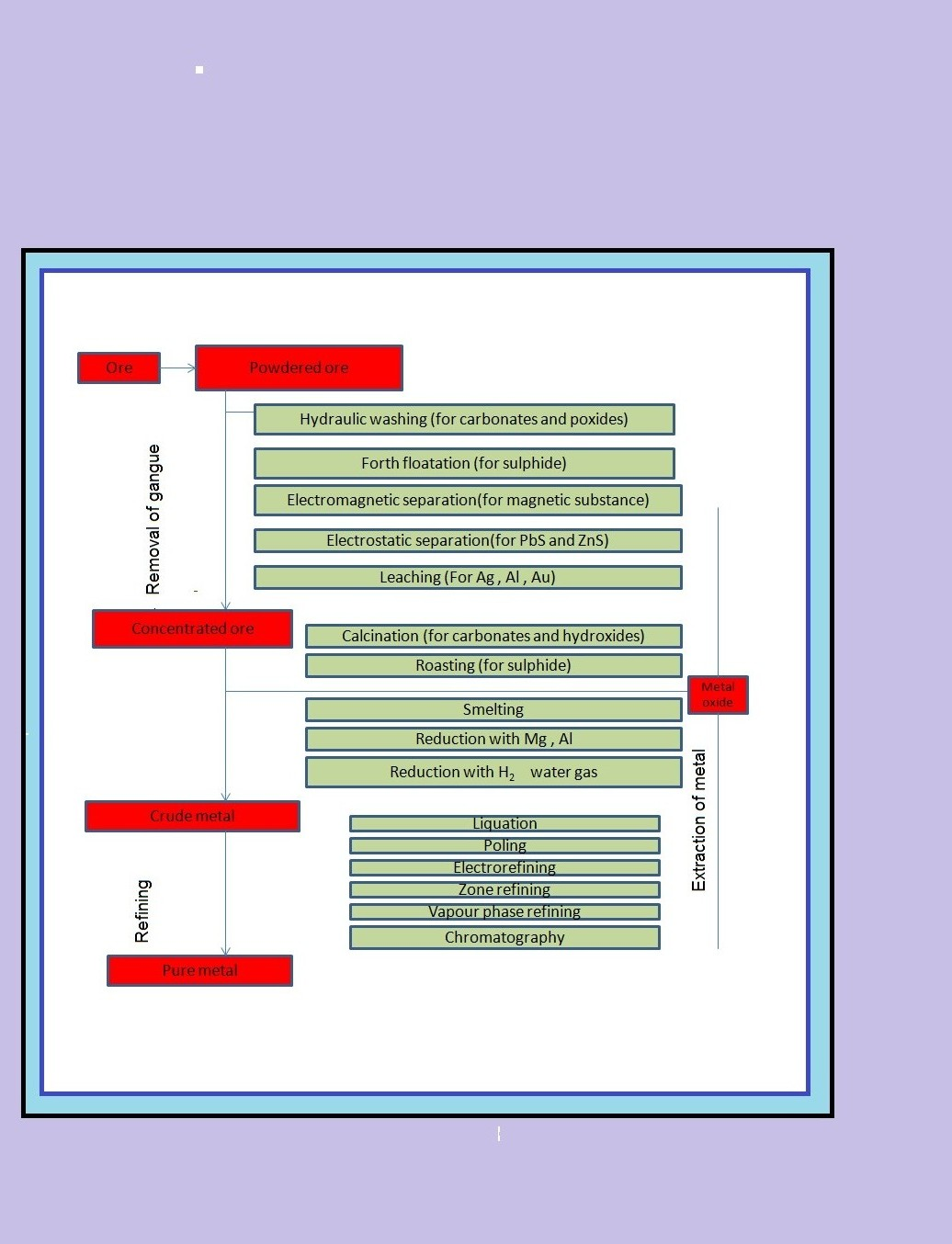
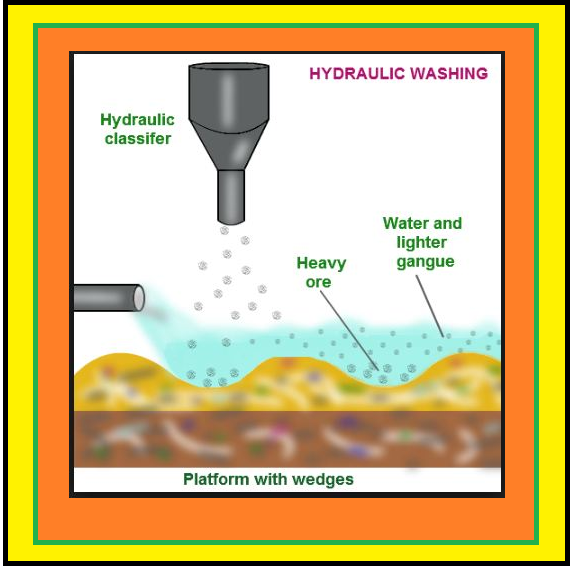
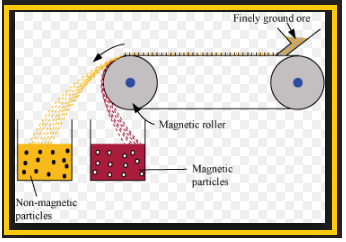
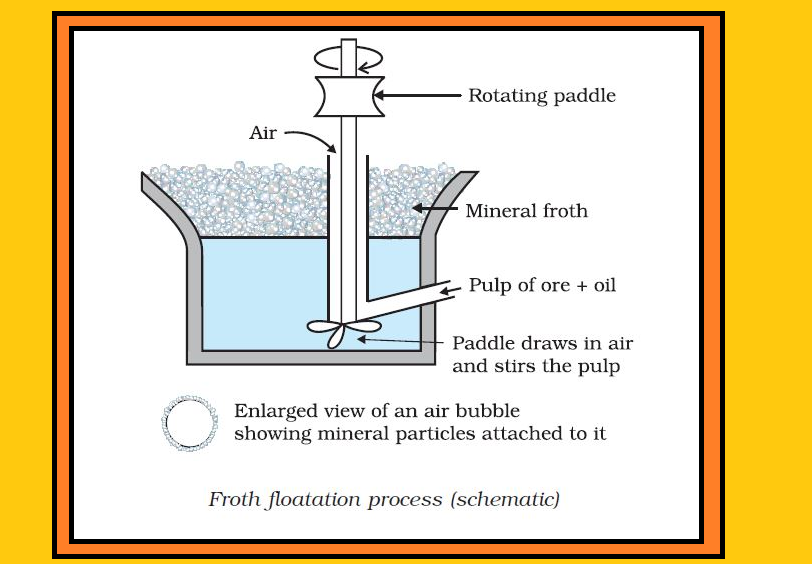
`=>` The concentrated ore must be converted into a form which is suitable for reduction.
`=>` Usually the sulphide ore is converted to oxide before reduction.
`=>` Oxides are easier to reduce.
`=>` Thus, isolation of metals from concentrated ore involves two major steps viz.,
(a) conversion to oxide, and
(b) reduction of the oxide to metal.
(a) `text(Conversion to Oxide :)`
(i) `text(Calcination :)` Calcinaton involves heating when the volatile matter escapes leaving behind the metal oxide.
`Fe_2O_3 * x H_2O (s) overset(Delta)→ Fe_2O_3 (s) +x H_2O (g)` ............(6)
`ZnCO_3(s) overset(Delta)→ ZnO (s) +CO_2 (g)` ...........(7)
`CaCO_3 . Mg CO_3 (s) overset(Delta ) → CaO(s) +2CO_2 (g)` ...........(8)
(ii) `text(Roasting :)` In roasting, the ore is heated in a regular supply of air in a furnace at a temperature below the melting point of the metal. Some of the reactions involving sulphide ores are :
`2ZnS +3O_2 → 2ZnO +2SO_2` ............(9)
`2PbS +3O_2 → 2PbO +2SO_2` ..........(10)
`2Cu_2S +3O_2 → 2Cu_2O +2SO_2` ........(11)
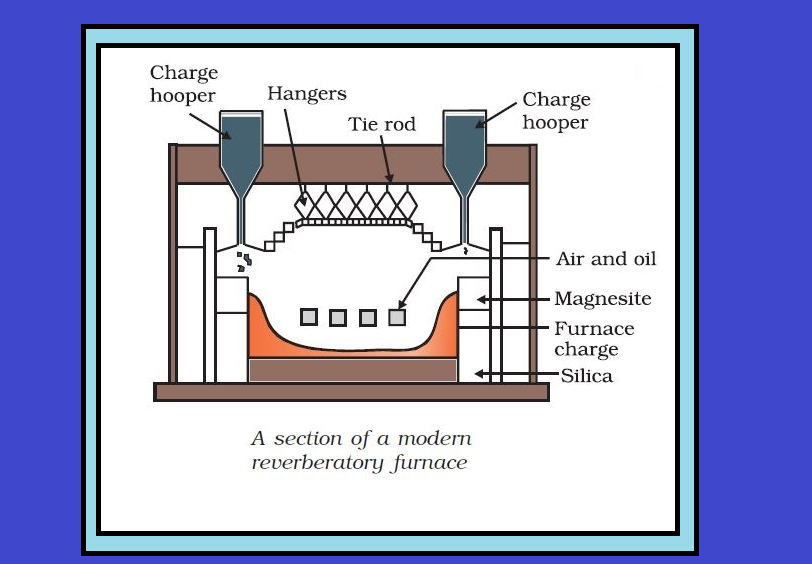
`=>` The sulphide ores of copper are heated in reverberatory furnace.
`=>` If the ore contains iron, it is mixed with silica before heating.
`=>` Iron oxide slags of as iron silicate and copper is produced in the form of copper matte which contains `Cu_2S` and `FeS`.
`=>` During metallurgy, ‘flux’ is added which combines with ‘gangue’ to form ‘slag’. Slag separates more easily from the ore than the gangue. This way, removal of gangue becomes easier.
`text(Flux) `: It is a substance that chemically combines with gangue (earthy impurities) which may still be present in the roasted or the caleined are to form an easily fusible material called the slag.
`text(Flux) +text(Gangue) → text(Slag)`
The slag thus formed melts at the temperature of the furnace . It is insoluble in the molten metal and also being lighter floats over the surface of the molten metal from where it can be skimmed off from time to time .
`text(Types of fluxes)` : Depending upon the nature of the impurities present in the ore, fluxes are classified the following two types :
`text(Acidic fluxes)`. For basic impurities like lime or metallic oxides `(CaO , FeO, MnO, etc)` present in the ore , acidic fluxes like silica `(SiO_2)` and borax `(Na_2B_4O_7. 10H_2O)` etc, are used .
`tt( (CaO , + , SiO_2 , → , CaSiO_3) , (FeO , + , SiO_2 , → , FeSiO_3) , (text{(basic impurities)} , , text{(Acidic flux)} , , text{(Fusible slag)}))`
`text(Basic fluxes)` . For acidic impurities like silica `(SiO_2)` , phosphorus pentoxide `(P_4O_(10))` etc. present in the ore , basic fluxes like limestone `(CaCO_3)` , magnesite `(MgCO_3)` , haematite `(Fe_3O_4)` etc. are used.
`tt((SiO_2 , + , CaCO_3 , → , CaSiO_3 , + , CO_2 ↑) , (SiO_2 , + , MgCO_3 , → , MgSiO_3 , + , CO_2 ↑) , (text{(Acidic impurities)} , , text{(Basic flux)} , , text{(Fusible slag)} , , ))`
`FeO+SiO_2 → underset(Slag)(FeSiO_3)` ..........(12)
The `SO_2` produced is utilised for manufacturing `H_2SO_4 .`
(b) `text(Reduction of Oxide to the Metal :)` Reduction of the metal oxide usually involves heating it with some other substance acting as a reducing agent (`C` or `CO` or even another metal). The reducing agent (e.g., carbon) combines with the oxygen of the metal oxide.
`M_x O_y + y C → x M +y CO` ...........(13)
`=>` Some metal oxides get reduced easily while others are very difficult to be reduced (reduction means electron gain or electronation). In any case, heating is required.
To understand the variation in the temperature requirement for thermal reductions (pyrometallurgy) and to predict which element will suit as the reducing agent for a given metal oxide (`M_xO_y)`, Gibbs energy interpretations are made.
`=>` The concentrated ore must be converted into a form which is suitable for reduction.
`=>` Usually the sulphide ore is converted to oxide before reduction.
`=>` Oxides are easier to reduce.
`=>` Thus, isolation of metals from concentrated ore involves two major steps viz.,
(a) conversion to oxide, and
(b) reduction of the oxide to metal.
(a) `text(Conversion to Oxide :)`
(i) `text(Calcination :)` Calcinaton involves heating when the volatile matter escapes leaving behind the metal oxide.
`Fe_2O_3 * x H_2O (s) overset(Delta)→ Fe_2O_3 (s) +x H_2O (g)` ............(6)
`ZnCO_3(s) overset(Delta)→ ZnO (s) +CO_2 (g)` ...........(7)
`CaCO_3 . Mg CO_3 (s) overset(Delta ) → CaO(s) +2CO_2 (g)` ...........(8)
(ii) `text(Roasting :)` In roasting, the ore is heated in a regular supply of air in a furnace at a temperature below the melting point of the metal. Some of the reactions involving sulphide ores are :
`2ZnS +3O_2 → 2ZnO +2SO_2` ............(9)
`2PbS +3O_2 → 2PbO +2SO_2` ..........(10)
`2Cu_2S +3O_2 → 2Cu_2O +2SO_2` ........(11)
`=>` The sulphide ores of copper are heated in reverberatory furnace.
`=>` If the ore contains iron, it is mixed with silica before heating.
`=>` Iron oxide slags of as iron silicate and copper is produced in the form of copper matte which contains `Cu_2S` and `FeS`.
`=>` During metallurgy, ‘flux’ is added which combines with ‘gangue’ to form ‘slag’. Slag separates more easily from the ore than the gangue. This way, removal of gangue becomes easier.
`text(Flux) `: It is a substance that chemically combines with gangue (earthy impurities) which may still be present in the roasted or the caleined are to form an easily fusible material called the slag.
`text(Flux) +text(Gangue) → text(Slag)`
The slag thus formed melts at the temperature of the furnace . It is insoluble in the molten metal and also being lighter floats over the surface of the molten metal from where it can be skimmed off from time to time .
`text(Types of fluxes)` : Depending upon the nature of the impurities present in the ore, fluxes are classified the following two types :
`text(Acidic fluxes)`. For basic impurities like lime or metallic oxides `(CaO , FeO, MnO, etc)` present in the ore , acidic fluxes like silica `(SiO_2)` and borax `(Na_2B_4O_7. 10H_2O)` etc, are used .
`tt( (CaO , + , SiO_2 , → , CaSiO_3) , (FeO , + , SiO_2 , → , FeSiO_3) , (text{(basic impurities)} , , text{(Acidic flux)} , , text{(Fusible slag)}))`
`text(Basic fluxes)` . For acidic impurities like silica `(SiO_2)` , phosphorus pentoxide `(P_4O_(10))` etc. present in the ore , basic fluxes like limestone `(CaCO_3)` , magnesite `(MgCO_3)` , haematite `(Fe_3O_4)` etc. are used.
`tt((SiO_2 , + , CaCO_3 , → , CaSiO_3 , + , CO_2 ↑) , (SiO_2 , + , MgCO_3 , → , MgSiO_3 , + , CO_2 ↑) , (text{(Acidic impurities)} , , text{(Basic flux)} , , text{(Fusible slag)} , , ))`
`FeO+SiO_2 → underset(Slag)(FeSiO_3)` ..........(12)
The `SO_2` produced is utilised for manufacturing `H_2SO_4 .`
(b) `text(Reduction of Oxide to the Metal :)` Reduction of the metal oxide usually involves heating it with some other substance acting as a reducing agent (`C` or `CO` or even another metal). The reducing agent (e.g., carbon) combines with the oxygen of the metal oxide.
`M_x O_y + y C → x M +y CO` ...........(13)
`=>` Some metal oxides get reduced easily while others are very difficult to be reduced (reduction means electron gain or electronation). In any case, heating is required.
To understand the variation in the temperature requirement for thermal reductions (pyrometallurgy) and to predict which element will suit as the reducing agent for a given metal oxide (`M_xO_y)`, Gibbs energy interpretations are made.